User`s guide
Contents ▲ 39 ▼ Index
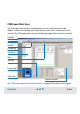
Validation Context
The Validation context is used to run and document qualification tests.
For both the bioanalyzer hardware and software tests can be run for:
• Installation qualification (IQ)
• Operational qualification (OQ)
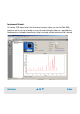
Validation results are automatically saved in .xvd files. You can re-open .xvd files to
review validation results.
For details, refer to “Performing Qualifications” on page 312.