User Manual Part 4
B-16
CLINICAL STUDY - COMPANION
Table B-5. Patient population characteristics for COMPANION (O PT and CRT-D)
Characteristic OPT
(N = 308)
CRT-D
(N = 595)
P-value
Age (years) Mean ± SD
66.7 ± 10.7 65.6 ± 11.2 0.14
Female
97 (31.4) 194 (32.6)
0.73
Gender [N (%)]
Male
211 (68.5) 401 (67.3)
0.73
Class III 253 (82.1) 512 (86.1)
0.12
NYHA Classification [N (%)]
Class IV 55 (17.8) 83 (13.9)
0.12
Ischemic 58.7 54.6 0.13
Ischemic Etiology (%)
Non-ischemic 41.3 45.4 0.13
LVEF (%) Mean ± SD
22.8 ± 7.2 22.5 ± 6.8 0.47
Resting Heart Ra te (bpm) Mean ± SD
72 ± 12 73 ± 13 0.37
QRS Width (ms) Mean ± SD
156 ± 24 159 ± 24 0.09
LBBB 69.8 72.9 0.21
Non-specific
21.4 16.8 0.21
Conduction Abnormality (%)
RBBB 8.77 10.2 0.21
Duration of Heart Failure (years) Mean ± SD
4.86 ± 4.41 4.44 ± 3.83 0.43
Diuretic 94.4 96.6 0.12
ACE inhibitor or ARB
88.6 89.6 0.66
Beta Blockers 66.2 67.6 0.69
Aldosterone Antagonist 54.8 55.1 0.94
Heart Failure Medications [(%)]
Digoxin 67.2 70.9 0.25
PATIENT ACCOUNTABILITY A ND FOLLOW-UP DURATION
The COMPANION study enrolled 1638 patients, with 1520 patients randomized
to a treatment group and one hundred eighteen patients (118) not randomized
due to changes in patient condition or consent between time of enrollment and
time of randomization, such that the inclusion criteria were no longer satisfied.
Of the 1520 patients, 595 were randomized to CRT-D with a mean follow-up of
1.3 years and 308 were randomized to OPT with a mean follow-up of 1.1 years.
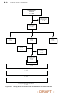
Figure B-1 on page B-18 provides an overview of patient enrollment.
Table B-6 on page B-17 gives a summary (by treatment group) of patient
disposition over time through 12 months after randomization. This does not
- DRAFT -