User Manual Part 4
CLINICAL STUDY - COMPANION
B-19
Event Contributing to Primary Endpoint and Secondary Endpoint of
All-cause Mortali ty
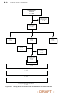
A total of 903 COMPANION patients in the CRT-D (595) and OPT (308) groups
were eligible for the primary endpoint. Figure B-2 on page B-19 provides patient
randomization and status for the primary endpoint and Figure B-3 on page B-19
provides patient randomization and status for the secondary mortality endpoint.
Randomized
n = 903 of 1520
OPT
n = 308
CRT-P
n = 595
Hospitalization
n = 196
Death
n = 20
Unknown
n = 15
No event
n = 77
Hospitalization
n = 372
Death
n = 18
Unknown
n = 5
No event
n = 200
No event
n = 92
Event
n = 216
No event
n = 205
Event
n = 390
Figure B-2. CRT-D and OPT patient randomization for primary endpoi nt
Randomized
n = 903
Alive
n = 218
Unknown
n = 15
Dead
n = 77
Alive
n = 484
Unknown
n = 6
Dead
n = 105
OPT
n = 308
CRT-D
n = 595
Figure B-3. CRT-D and OPT patient randomization for mortality endpoint
- DRAFT -