Use Instructions
2
/
11
INTENDED USE
The PocketECG transmitter is intended to
acquire,
analyze,
visualize, record or/and transmit the ECG
acceleration data. The results of arrhythmia
and ST elevation detection are displayed, stored
or/and transmitted along with
the ECG signals. The
acceleration signals are analyzed in order to
determine the physical activity of
the patient. It is
assumed that the device can further transmit the
and acceleration signals along with analysis
results using available wireless technologies.
The
PocketECG IV is intended for use under the
supervision of a
physician or those knowledgeable
all aspects of ECG morphology, rhythm, and
arrhythmias. Having
fulfilled the working conditions
specified in the manual, the device may be used
when the patient is
in the following places: clinic,
hospital, outpatient cardiology
clinic, house,
business establishment, etc.
CONTRAINDICATIONS
The
PocketECG IV is not intended to be used by
patients who have been diagnosed with
threatening arrhythmias and require
spitalization or
patients who require inpatient
monitoring using a
life-saving device.
The Pocket ECG III is not intended for use in surgical
rooms, intensive care units, intermediate or
step
-down units, emergency vehicles. The
PocketECG IV
is MR unsafe and should not be used
in any magnetic resonance environment.
COMPLIANCE
The Medicalgorithmics Unified Arrhythmia
Diagnostic System
PocketECG IV complies with the
following regulations
:
the essential requirements of the Council
Directive 93/42/EEC,
the
requirements of the United States Food and
Drugs Administration,
the requirements of the Health Canada Medical
Devices Regulations.
US Federal Law restricts this device to sale by or on
the order of a physician.
FCC REQUIREMENTS
FCC ID:
2AB2MP4TRAA
The device complies with part 15 of the FCC Rules.
Operation is subject to the following two
conditions: (1) This device may not cause harmful
interference, and (2) this device must accept any
interference received, including interference that
may cause undesired operation.
Changes or modifications of any kind not expressly
approved by Medicalgorithmics S.A. could void the
user's authority to use ECG.
COMPONENTS & ACCESSORIES
The PocketECG transmitter, model:
PocketECG IV, type: P4TR-AA-ADS (LTE version
for Verizon), P4TR-BA-ADS (LTE version for
AT&T)
A Lithium-ion battery, type: PECGB-III, rated
3,7 VDC, 1700 mAh
An AC plug-in charger type PECGC-III suitable
for charging battery type PECGB-III,
manufactured by Medicalgorithmics S.A., rated
100 VAC – 240 VAC, 0.2 A, 50/60 Hz
MicroSD memory card
PocketECG pouch
Holster (optional, for indoor use only)
Accessories which are needed to the proper
operation but are not included in the PocketECG IV
package:
• SIM card,
• ECG electrodes.
1
2
3
1
3
2
11
/
11
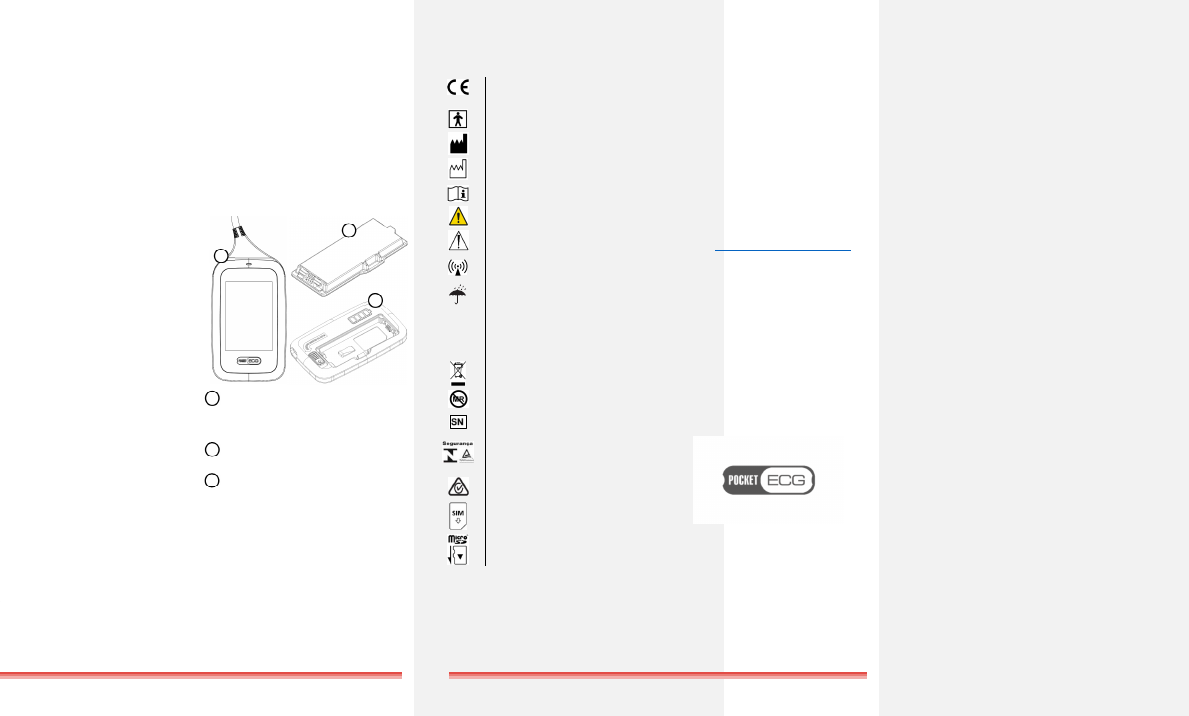
SYMBOLS USED ON POCKETECG
TRANSMITTER CASING
0197
CE mark (Council Directive 93/42/EEC)
Type BF applied part
Manufacturer’s name and address
Date of manufacture
Consult instructions for use
Warning
Caution
PocketECG transmitter includes radio
wave transmitters
Keep dry
IP20
Protection against solid particles up to
12.5 mm (fingers or similar objects),
lack of protection against ingress of
water
Dispose the device in compliance
with appropriate regulations
MR unsafe
Serial number
Brazilian National Institute of
Metrology, Quality and Technology
(Inmetro) certification mark
Australian Communications and Media
Authority (RCM) certification mark
SIM card (GSM)
MicroSD memory card
MEDICAL INCIDENT, SERVICE, DECLARATION
OF CONFORMITY
• In the event of a medical incident, please notify
the manufacturer immediately.
• If you would like to receive the declaration of
conformity, please contact the manufacturer.
• Service is provided only by Medicalgorithmics
S.A. In case of any product malfunction it shall be
returned directly to manufacturer to
the following address:
M
EDICALGORITHMICS
S.A.
Aleje Jerozolimskie 81
02-001 Warsaw, Poland
e-mail: technical@medicalgorithmics.com
COPYRIGHTS
Copyright 2018 Medicalgorithmics S.A. No one is
permitted to reproduce or duplicate, in any form,
this instructions for use or any part thereof without
permission from Medicalgorithmics S.A.
Medicalgorithmics S.A assumes no responsibility
for any injury or for any illegal or improper use of
the product that may result from failure to use this
product in accordance with the instructions,
cautions, warnings, or statement of intended use
published in this manual.