Certifications 2
SDS No.: 528149 V002.0
Pattex Repair 100% universal adhesive
Page 10 of 16
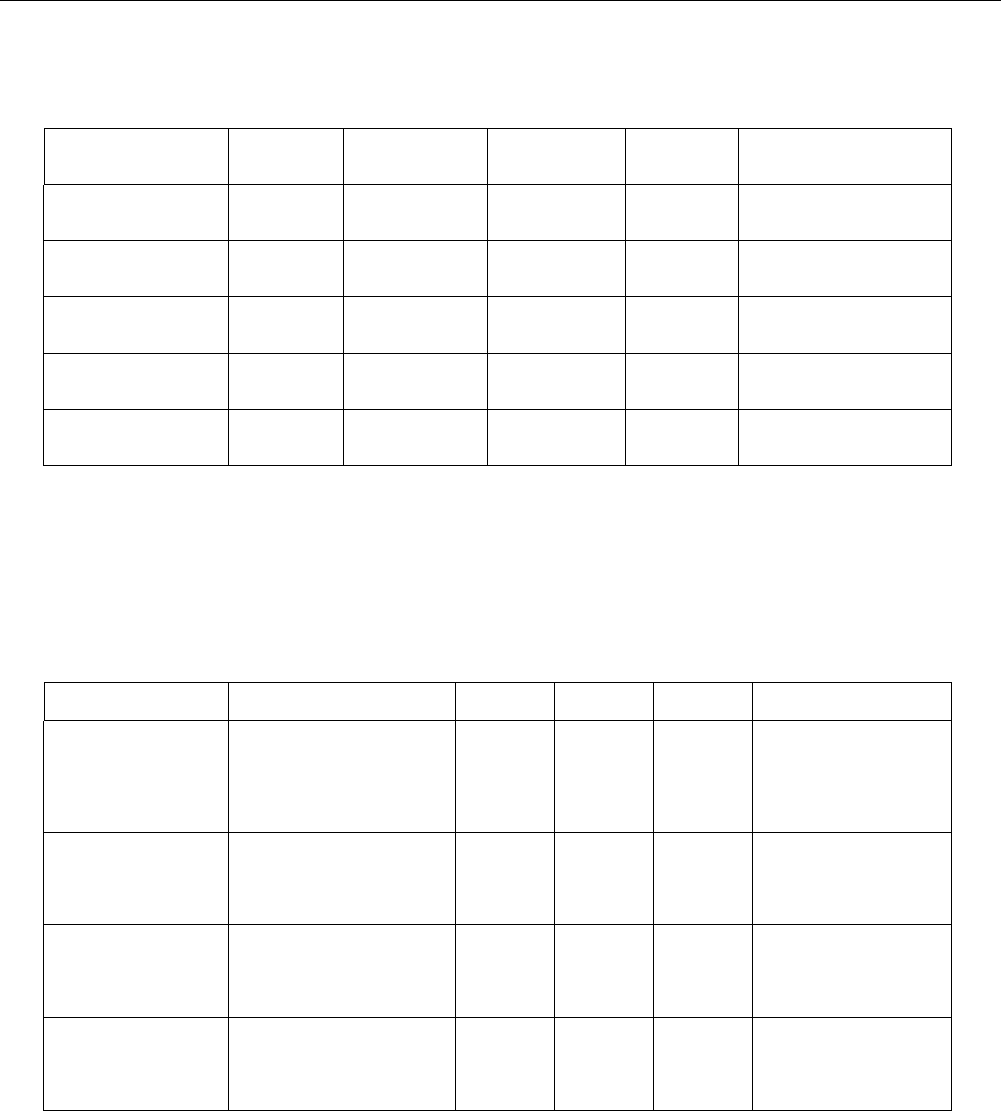
Germ cell mutagenicity:
The mixture is classified based on threshold limits referring to the classified substances present in the mixture.
Hazardous substances
CAS-No.
Result
Type of study /
Route of
administration
Metabolic
activation /
Exposure time
Species
Method
Benzene, C10-13-alkyl
derivs.
67774-74-7
negative
bacterial reverse
mutation assay (e.g
Ames test)
with and without
EU Method B.13/14
(Mutagenicity)
Benzene, C10-13-alkyl
derivs.
67774-74-7
negative
mammalian cell
gene mutation assay
with and without
OECD Guideline 476 (In vitro
Mammalian Cell Gene
Mutation Test)
Trimethoxyvinylsilane
2768-02-7
negative
bacterial reverse
mutation assay (e.g
Ames test)
with and without
OECD Guideline 471
(Bacterial Reverse Mutation
Assay)
Trimethoxyvinylsilane
2768-02-7
positive
in vitro mammalian
chromosome
aberration test
with and without
OECD Guideline 473 (In vitro
Mammalian Chromosome
Aberration Test)
Trimethoxyvinylsilane
2768-02-7
negative
mammalian cell
gene mutation assay
with and without
OECD Guideline 476 (In vitro
Mammalian Cell Gene
Mutation Test)
Carcinogenicity
No data available.
Reproductive toxicity:
The mixture is classified based on threshold limits referring to the classified substances present in the mixture.
Hazardous substances
CAS-No.
Result / Value
Test type
Route of
application
Species
Method
Benzene, C10-13-alkyl
derivs.
67774-74-7
NOAEL P >= 50 mg/kg
NOAEL F1 >= 50 mg/kg
NOAEL F2 >= 50 mg/kg
Two
generation
study
oral: gavage
rat
OECD Guideline 416 (Two-
Generation Reproduction
Toxicity Study)
Trimethoxyvinylsilane
2768-02-7
NOAEL P 250 mg/kg
one-
generation
study
oral: gavage
rat
OECD Combined Repeated
Dose and Reproductive /
Developmental Toxicity
Screening Test (Precursor
Protocol of GL 422)
Trimethoxyvinylsilane
2768-02-7
NOAEL P 1.000 mg/kg
one-
generation
study
oral: gavage
rat
OECD Combined Repeated
Dose and Reproductive /
Developmental Toxicity
Screening Test (Precursor
Protocol of GL 422)
Trimethoxyvinylsilane
2768-02-7
NOAEL F1 1.000 mg/kg
one-
generation
study
oral: gavage
rat
OECD Combined Repeated
Dose and Reproductive /
Developmental Toxicity
Screening Test (Precursor
Protocol of GL 422)
STOT-single exposure:
No data available.










