Use Instructions

REGISTRATION TRAY
TMRW-SPC-CUS-006 • VERSION: 01 • IMPLEMENTATION DATE: DD MMM YYYY
INSTRUCTIONS FOR USE TMRW REGISTRATION TRAY
TMRW REGISTRATION TRAY
Cryopreservation Identification and Organizational Device
DEVICE DESCRIPTION
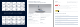
The TMRW Registration Tray is an RFID registering site
for cryodevices and their associated CryoBeacons.
INTENDED USE
To provide users with an RFID enabled tool for use during
the registration of containers.
INTENDED USERS
The intended users of the Registration Tray are
laboratory professionals including embryologists,
andrologists, clinicians, laboratory technicians, and
laboratory management personnel.
Patients do not interact with or come into contact with
TMRW products.
The patient population served is men and women,
typically age between the ages of 18 years to 50
years at the time of fertility tissue collection.
CONTRAINDICATIONS
None.
ADVERSE EFFECTS
None.
STORAGE
Store at room temperature.
DISPOSAL
Discard in a e-waste facility.
There are no lithium batteries used to operate, nor are
any toxic components used in the manufacturing of the
Registration Tray. The PCB assembly is lead-free.
Always use appropriate personal protective equipment (PPE) and follow your laboratory’s standard operating
procedures and safety guidelines when working with liquid nitrogen (LN
2
).
The Registration Tray requires electricity to function. Use standard caution and care around electrical connections
and cords to prevent bodily harm from electrical shock or damage to the machine.
Always use proper power and proper electrical compliant connections in accordance with the local electrical code.
Place the CryoBeacon in the leftmost slot in the Registration Tray.
Label the cryodevices.
Place the labelled cryodevices in the paired CryoBeacon.
Cap the CryoBeacon.
Move the CryoBeacon to the one of the four slots on the right side of the Registration Tray.
01
02
03
04
05
INSTRUCTIONS FOR USE
Figure A: Registration Tray